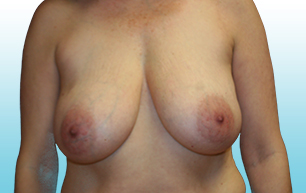
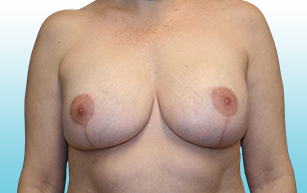
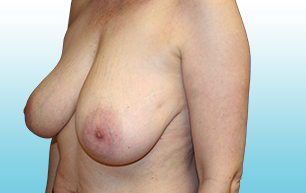
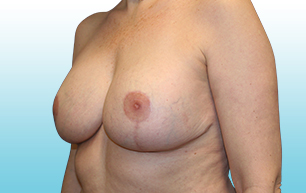
Breast implants are not considered lifetime devices. The longer people have them, the greater the chances are that they will develop complications, some of which will require more surgery.
Breast implants have been associated with the development of a cancer of the immune system called breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). This cancer occurs more commonly in patients with textured breast implants than smooth implants, although rates are not well defined. Some patients have died from BIA-ALCL.
Patients receiving breast implants have reported a variety of systemic symptoms, such as joint pain, muscle aches, confusion, chronic fatigue, autoimmune diseases, and others. Individual patient risk for developing these symptoms has not been well established. Some patients report complete resolution of symptoms when the implants are removed without replacement.
Natrelle® Breast Implants are approved for the following:
-
Breast augmentation for women at least 22 years old for silicone-filled implants and for women at least 18 years old for saline-filled implants.
Breast augmentation includes primary breast augmentation to increase the breast size and revision surgery to correct or improve the result of a primary breast augmentation
-
Breast reconstruction. This includes primary breast reconstruction to replace breast tissue that has been removed due to cancer or trauma or that has failed to develop properly due to a severe breast abnormality. This also includes revision surgery to correct or improve the result of a primary breast reconstruction
Breast implant surgery should NOT be performed in:
- Women with active infection anywhere in their body
- Women with existing cancer or precancer of their breast who have not received adequate treatment for those conditions
- Women who are currently pregnant or nursing
Tell your doctor if you have any of the following conditions, as the risks of breast implant surgery may be higher:
- Autoimmune diseases (eg, lupus and scleroderma)
- A weakened immune system (eg, taking medications to decrease the body’s immune response)
- Planned chemotherapy or radiation therapy following breast implant placement
- Conditions or medications that interfere with wound healing and blood clotting
- Reduced blood supply to breast tissue
- Clinical diagnosis of depression or other mental health disorders, including body dysmorphic disorder and eating disorders
- Those with a diagnosis of depression or other mental health disorders should wait for resolution or stabilization of these conditions prior to undergoing breast implantation surgery
- There is a Boxed Warning for breast implants. Please see bold text at beginning
- Many changes to your breasts following implantation are irreversible. If you later choose to have your implants removed and not replaced, you may experience dimpling, puckering, wrinkling, or other cosmetic changes, which may be permanent
- Breast implantation is likely not a one-time surgery. The longer implants are in place, the greater the potential risk for complications. You will likely need additional surgeries on your breasts due to complications or unacceptable cosmetic results. Thus, you should also consider the complication rates for later (revision) surgery since you may experience these risks in the future
- Cancer treatments and surgery will affect the outcome and timing of breast reconstruction
- Breast implants may affect your ability to breastfeed, either by reducing or eliminating milk production
- Rupture of a silicone-filled breast implant is most often silent. Even if you have no symptoms, you should have your first ultrasound or MRI at 5 to 6 years after your initial implant surgery and then every 2 to 3 years thereafter regardless of whether your implants are for augmentation or reconstruction. If you have symptoms of or uncertain ultrasound results for breast implant rupture, an MRI is recommended. Additional imaging may be required depending on your medical history and status. The health consequences of a ruptured silicone gel-filled breast implant have not been fully established
- Routine screening mammography for breast cancer will be more difficult, and implants may rupture during the procedure. Perform self-examination every month for cancer screening and ask your surgeon to help you distinguish the implant from your breast tissue. Lumps, persistent pain, swelling, hardening, or changes in implant shape should be reported to your surgeon and possibly evaluated with imaging
Key complications include reoperation, implant removal with or without replacement, implant rupture with silicone-filled implants, implant deflation with saline-filled implants, and capsular contracture (severe scar tissue around the implant). Other complications include breast pain, swelling, asymmetry, wrinkling/rippling, implant malposition nipple complications, hypertrophic scarring, and implant palpability/visibility.
Talk to your doctor about other complications.
For more information, see the patient brochures at rxabbvie.com.
To report a problem with Natrelle® Breast Implants, please call Allergan® at 1-800-624-4261.
The sale and distribution of Natrelle® Breast Implants is restricted to licensed physicians who provide information to patients about the risks and benefits of breast implant surgery.
Natrelle® 133S Tissue Expanders are approved for breast reconstruction following mastectomy, treatment of underdeveloped breasts, and treatment of soft tissue deformities.
Do not use if you:
- Already have implanted devices that would be affected by a magnetic field
- Have tissue unsuitable for expansion
- Have an active infection or a residual gross tumor at the expansion site
- Are undergoing adjuvant radiation therapy
- Have a physiological condition (eg, obesity, smoking, diabetes, autoimmune disease, hypertension, chronic lung or severe cardiovascular disease, or osteogenesis imperfecta) or use certain drugs (including those that interfere with blood clotting or affect tissue viability) that may result in a high risk of surgical and/or postoperative complications
- Natrelle® 133S Tissue Expanders should NOT be used in patients who already have implanted devices that would be affected by a magnetic field
- Active infection anywhere may increase risk of infection around the tissue expander. Certain infections may require premature removal of the device
- Natrelle® 133S Tissue Expanders are temporary devices and are not to be used for permanent implantation or beyond 6 months. Tissue expansion in breast reconstruction typically requires 4 months to 6 months
Deflation, tissue damage and/or appearance of the implant through the skin, infection, unwanted shape, unintended blood or fluid collection, capsular contracture (tightening of scar tissue that causes the breast to harden), premature device removal, bone/pain/sensation changes, and inflammation.
Natrelle® 133S Tissue Expanders are available by prescription only.
The sale and distribution of Natrelle® 133S Tissue Expanders is restricted to licensed physicians.
IMPORTANT SAFETY INFORMATION
BOTOX® Cosmetic may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of BOTOX® Cosmetic:
-
Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
-
Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, and trouble swallowing.
BOTOX®
Cosmetic dosing units are not the same as, or comparable to, any other botulinum toxin product.
There has not been a confirmed serious case of spread of toxin effect when BOTOX® Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.
BOTOX® Cosmetic may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX® Cosmetic.
If this happens, do not drive a car, operate machinery, or do other dangerous activities.
Serious and/or immediate allergic reactions have been reported. They include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Get medical help right away if you are wheezing or have asthma symptoms, or if you become dizzy or faint.
Do not receive BOTOX® Cosmetic if you: are allergic to any of the ingredients in BOTOX® Cosmetic (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA); have a skin infection at the planned injection site.
Tell your doctor about all your muscle or nerve conditions, such as ALS or Lou Gehrig’s disease, myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including difficulty swallowing and difficulty breathing from typical doses of BOTOX® Cosmetic.
Tell your doctor about all your medical conditions, including: plans to have surgery; had surgery on your face; have trouble raising your eyebrows; drooping eyelids; any other abnormal facial change; are pregnant or plan to become pregnant (it is not known if BOTOX® Cosmetic can harm your unborn baby); are breast-feeding or plan to (it is not known if BOTOX® Cosmetic passes into breast milk).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using BOTOX® Cosmetic with certain other medicines may cause serious side effects.
Do not start any new medicines until you have told your doctor that you have received BOTOX® Cosmetic in the past.
Tell your doctor if you have received any other botulinum toxin product in the last 4 months; have received injections of botulinum toxin such as Myobloc®, Dysport®, or Xeomin® in the past (tell your doctor exactly which product you received); have recently received an antibiotic by injection; take muscle relaxants; take an allergy or cold medicine; take a sleep medicine; take aspirin-like products or blood thinners.
Other side effects of BOTOX® Cosmetic include: dry mouth; discomfort or pain at the injection site; tiredness; headache; neck pain; and eye problems: double vision, blurred vision, decreased eyesight, drooping eyelids and eyebrows, swelling of your eyelids and dry eyes.
For more information refer to the Medication Guide or talk with your doctor.
JUVÉDERM® Injectable Gel Fillers Important Information
APPROVED USES
JUVÉDERM® VOLUX® XC injectable gel is for deep injection to improve moderate to severe loss of jawline definition in adults over the age of 21.
JUVÉDERM® VOLUMA® XC injectable gel is for deep injection in the cheek area to correct age-related volume loss and for augmentation of the chin region to improve the chin profile in adults over 21.
JUVÉDERM® VOLLURE® XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC injectable gels are for injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. JUVÉDERM® VOLLURE® XC injectable gel is for adults over 21.
JUVÉDERM® Ultra XC injectable gel is also for injection into the lips and perioral area for lip augmentation in adults over 21.
JUVÉDERM® VOLBELLA® XC injectable gel is for injection into the lips for lip augmentation and correction of perioral lines, and for injection into the undereye hollows to improve the appearance of undereye hollows in adults over the age of 21.
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive any JUVÉDERM® formulation?
Do not use these products if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), if you are allergic to lidocaine or the Gram-positive bacterial proteins used in these products, or if you have had previous allergic reactions to hyaluronic acid fillers.
What warnings should my doctor advise me about?
-
One of the risks with using dermal fillers is the unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. Most of these events are irreversible.
-
If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin, or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
-
The use of dermal fillers where skin sores, pimples, rashes, hives, cysts, or infections are present should be postponed, as this may delay healing or make skin problems worse.
-
The effectiveness of removal of any dermal filler has not been studied.
What precautions should my doctor advise me about?
-
JUVÉDERM® VOLBELLA® XC should only be injected into undereye hollows by doctors who have completed the necessary training for this treatment area. To find a doctor, visit Juvederm.com/find-a-specialist. Doctors who complete the training will be listed with a symbol
-
The safety of these products for use during pregnancy or while breastfeeding has not been studied
-
The safety of JUVÉDERM® VOLUMA® XC has not been studied in patients under 35 years or over 65 years for cheek augmentation, or under 22 years and over 80 years for chin augmentation. The safety of JUVÉDERM® VOLUX® XC, JUVÉDERM® VOLLURE® XC and JUVÉDERM® VOLBELLA® XC has not been studied in patients under 22 years, and the safety of JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC has not been studied in patients under 18 years
-
The safety and effectiveness of treatment with JUVÉDERM® products in anatomical regions outside of their approved uses have not been established in clinical studies
-
If you have a history of excessive scarring (thick, hard scars) or pigmentation disorders, treatment in these patients has not been studied and may result in additional scars or changes in pigmentation
-
If you are planning other procedures including laser treatments or a chemical peel, there is a possible risk of inflammation at the treatment site if these procedures are performed closely before or after JUVÉDERM® injectable gel treatment
-
Tell your doctor if you are on therapy used to reduce your body’s natural defense system (such as steroids, chemotherapy, and medicines to treat autoimmune diseases, HIV, and AIDs), as these may increase your risk of infection; and medications that can prolong bleeding (such as aspirin, ibuprofen, or other blood thinners), as these may result in increased bruising or bleeding at the injection site.
-
Avoid applying makeup for 12 hours after treatment and minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment, as these may cause temporary redness, swelling, and/or itching at the injection site
-
JUVÉDERM® VOLUMA® XC was not studied in patients with significant loose skin of the chin, neck, or jaw
-
The effect of JUVÉDERM® VOLUMA® XC injection into the chin on facial hair growth has not been studied
-
Patients who experience skin injury near the site of JUVÉDERM® VOLUMA® XC injection may be at a higher risk for adverse events
-
Tell your doctor if you have already been injected with dermal fillers in the same area as the one(s) you are about to be treated for. This information helps your doctor decide when and whether you should get treatment
What are possible side effects of treatment?
The most commonly reported side effects with JUVÉDERM® injectable gels were redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA® XC, dryness was also reported.
These side effects are consistent with other facial injection procedures and most will resolve within 30 days. Your doctor may choose to treat side effects persisting longer with antibiotics, steroids, or hyaluronidase (an enzyme that breaks down hyaluronic acid).
As with all skin injection procedures, there is a risk of infection.
To report a side effect with any product in the JUVÉDERM® Collection, please call the Allergan® Product Support Department at 1-877-345-5372. Please also visit Juvederm.com or talk to your doctor for more information.
Products in the JUVÉDERM® Collection are available only by a licensed physician or properly licensed practitioner.
Natrelle Perks® Terms and Conditions
- Allē Members who undergo breast augmentation with Natrelle® gel implants (“Member”) may qualify to receive either (a) one (1) complimentary treatment of BOTOX® Cosmetic (onabotulinumtoxinA) up to 50 units OR (b) one (1) complimentary treatment of JUVÉDERM® Ultra XC up to two .55 mL syringes at participating Allē provider offices only. Allergan Aesthetics is not responsible for any associated injection costs.
- Members enrolled in Medicare, Medicaid, or other federal or state healthcare programs are not eligible for this offer.
- Member must claim offer in the Allē app via text message link within 6 months of their Natrelle® breast augmentation and select their complimentary treatment.
- Once claimed, the selected complimentary treatment cannot be changed and the offer must be redeemed within 6 months of the offer being deposited into Member’s Allē Wallet. Offer expires 6 months after issue date into Allē Wallet.
- Limit 1 per Member.
-
A healthcare provider will determine if Member is an appropriate candidate for a BOTOX® Cosmetic or JUVÉDERM® Ultra XC treatment.
- If Member is an appropriate candidate, offer can be redeemed at a participating provider’s office.
- Standard Allē Loyalty Program Terms and Conditions apply.
-
Members will earn Allē points on all qualifying
Earnings Eligible Product purchases,
subject to applicable earnings caps.
- The value of this offer cannot be redeemed or exchanged for cash.
- Offer cannot be applied to past transactions.
- Offer cannot be combined with other Allē offers on BOTOX® Cosmetic or the JUVÉDERM® Collection of Fillers but can be combined with Allē and Allē brand-specific gift cards, Allē points offers, including Double Points offers, and other Allē brand-specific offers.
- The complete value of this offer must be used in a single transaction.
- If you have questions, please contact Allē Customer Support at 1-888-912-1572 Monday - Friday, 8 am – 6 pm CT.
- Allergan Aesthetics, an AbbVie company, reserves the right to alter or cancel this offer at any time.
Back to top